Ph3 Lewis Structure Shape
Draw a trial structure by putting electron pairs around every atom until each gets an octet. Count the valence electrons in your trial structure (8). Now, count the valence electrons you actually have available. 1 P + 3 H =. 1×5 + 3×1 = 8. The trial structure has exactly the same number of electrons as we have available.

PH3 Lewis StructureLewis Structure of PH3 (Phosphorus Trihydride)Draw Lewis Structure for PH3
Phosphine PH3 Lewis Dot Structure shadowboy220 1.9K subscribers 49K views 11 years ago Chemistry Lewis Dot Structures A video explanation of how to draw the Lewis Dot Structure for.

رباعي السطوح الهندسة الجزيئية الميثان جزيء ذرة ، رائحة باهتة من الغاز, متنوعة, زاوية, نص png
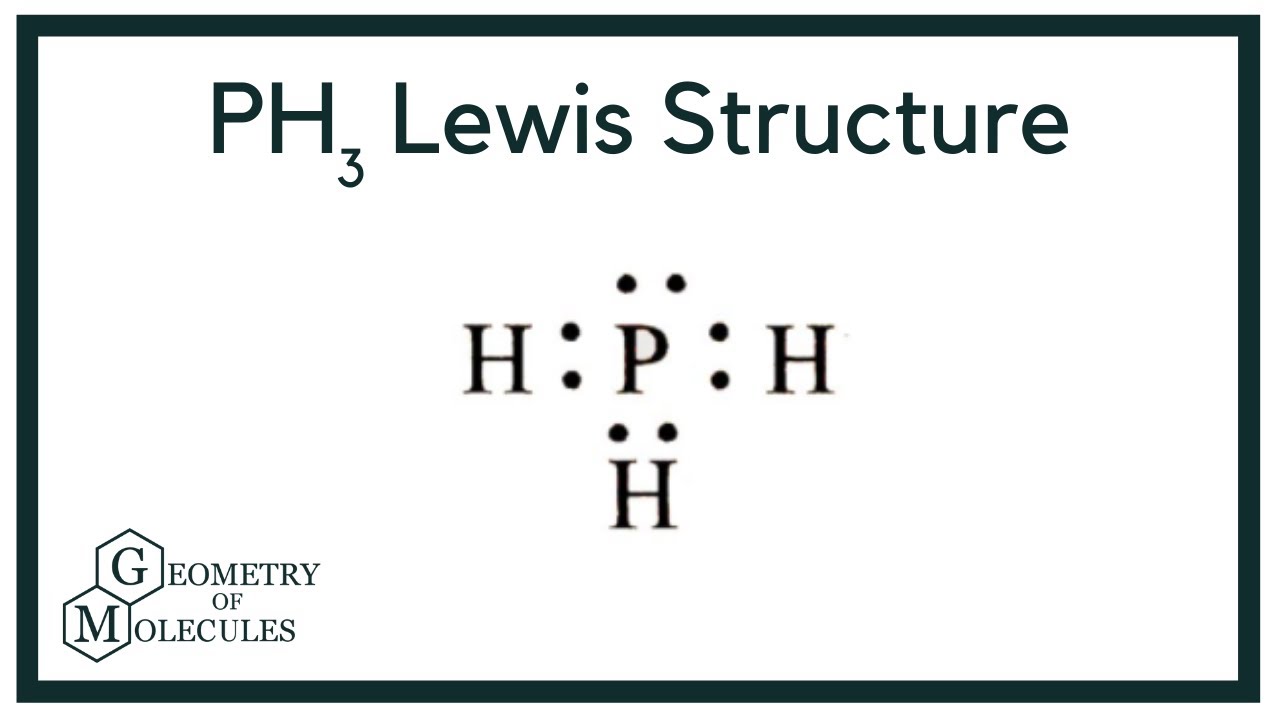
The Lewis structure for PH 3 is similar to NH 3. In the PH 3 Lewis structure (and all Lewis structures) hydrogen goes on the outside. Remember, too, that hydrogen only needs two valence electrons to have a full outer shell. In the Lewis structure for PH 3 there are a total of 8 valence electrons. Three pairs will be used in the chemical bonds.
PH3 Lewis Structure (Phosphine) YouTube
Lewis Structure is the pictorial representation of the arrangement of atoms and valence electrons in the molecule. To know the Lewis Structure, we first know the central atom and the arrangement of other atoms. Here for PH3, the phosphorus atom will take the central position as Hydrogen atoms cannot take a central position in the Lewis Structure.
Solved Part A Draw the Lewis structure of PH3 To add lone
Shape of PH 3 is trigonal pyramidal. Molecular geometry around phosphorous atom is tetrahedral. Total valence electrons pairs around phosphorous atom is four. In this tutorial we will learn how to draw the lewis structure of PH 3 and determining the shape and molecular geometry of the molecule. PH 3 lewis structure
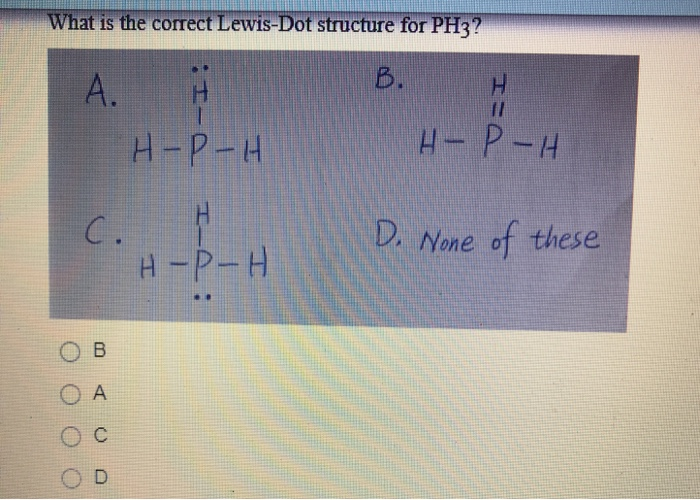
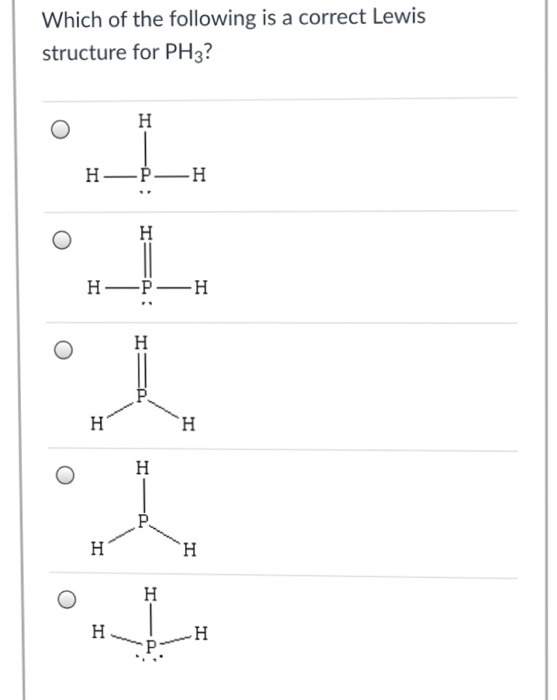
Solved What is the correct LewisDot structure for PH3? А. Н
PH3 Lewis Structure in 6 Steps (With Images) By Jay Rana / Last Updated On: June 21, 2023 So you have seen the above image by now, right? Let me explain the above image in short. PH3 lewis structure has a Phosphorus atom (P) at the center which is surrounded by three Hydrogen atoms (H).

Lewis Structure Practice Worksheet Lewis Dot Diagrams Chemistry Handout Answers Diagram Base
We will study the PH3 lewis structure and understand the concept. Some facts about Phosphane. PH3 has a molar mass equal to 33.99 g/mol. It exists as a gas that is colorless and has an odor like that of a rotten fish. The density of PH3 is 1.37 g/L. The melting point of the compound is said to be-132 degrees Celsius and the observed boiling.

Draw the Lewis dot structure for PH3 YouTube
From the Lewis molecular structure of PH3, we have seen the phosphorous atom has five valence electrons. During the bonding process, Phosphorous is surrounded by three hydrogen atoms, and each is connected by a single bond. The two remaining electrons form a lone pair.

How To Draw Electron Dot Diagrams Elementchampionship Jeffcoocctax
This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

Chemistry Class 11 NCERT Solutions Chapter 4 Chemical Bonding and Molecular Structure Part 2
A Lewis electron dot diagram (or electron dot diagram, or a Lewis diagram, or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the.
Chemistry Archive November 11, 2017
A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.

Lewis dot structure covalent bond ph3 Jennifer Reyes, Jenna Kriho Science ShowMe
The PH3 Lewis structure has 8 valence electrons. Remember that hydrogen (H) only needs two valence electrons to have a full outershell. The Lewis structure for PH3 is similar the the structure for NH3 since both P and N are in the same group on the Periodic table. PH3 Lewis Structure - How to Draw the Lewis Structure for PH3 It is helpful if you:

H Lewis Dot Structure
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

Ph3 Lewis Structure Shape
Phosphine (PH3) lewis dot structure, molecular geometry, hybridization, Bond angle, polar or nonpolar Phosphine is a very toxic and dangerous gas, it has a chemical formula of PH 3. It is a colorless gas and has an odor like fish or garlic.

[Punjabi] Draw lewis dot structure of PH3
Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion.

Ph3 Lewis Structure Shape
GENERAL TERMS FOR LEWIS DOT STRUCTURES: 1. Dot • one dot represents one valence electron (found on odd-electron particles). 2. Pair of Dots •• a pair of dots represents a nonbonding (lone) pair of electrons that are not involved in a covalent bond and "belong to" only one atom. 3. Dash each dash represents two electrons that are shared between two atoms as a covalent bond.